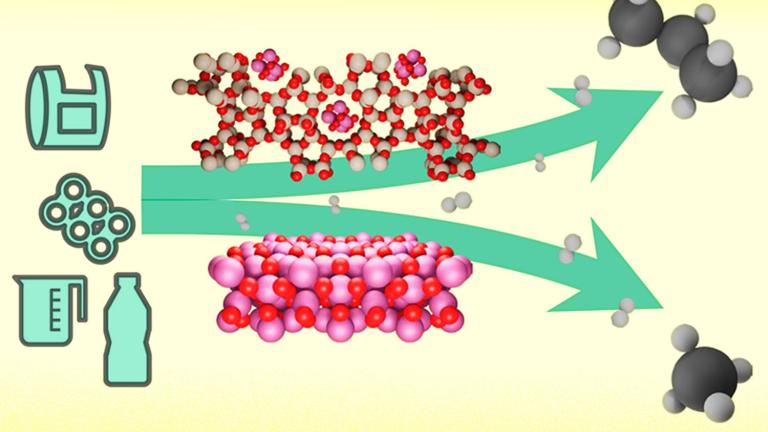
The accumulation of plastic waste in the oceans, soil, and even in our bodies is one of the major pollution issues of modern times, with over 5 billion tons disposed of so far. Despite major efforts to recycle plastic products, actually making use of that motley mix of materials has remained a challenging issue.
A key problem is that plastics come in so many different varieties, and chemical processes for breaking them down into a form that can be reused in some way tend to be very specific to each type of plastic. Sorting the hodgepodge of waste material, from soda bottles to detergent jugs to plastic toys, is impractical at large scale. Today, much of the plastic material gathered through recycling programs ends up in landfills anyway. Surely there’s a better way.
According to new research from MIT and elsewhere, it appears there may indeed be a much better way. A chemical process using a catalyst based on cobalt has been found to be very effective at breaking down a variety of plastics, such as polyethylene (PET) and polypropylene (PP), the two most widely produced forms of plastic, into a single product, propane. Propane can then be used as a fuel for stoves, heaters, and vehicles, or as a feedstock for the production of a wide variety of products — including new plastics, thus potentially providing at least a partial closed-loop recycling system.
The finding is described today in the open access journal JACS Au, in a paper by MIT professor of chemical engineering Yuriy Román-Leshkov, postdoc Guido Zichitella, and seven others at MIT, the SLAC National Accelerator Laboratory, and the National Renewable Energy Laboratory.
Recycling plastics has been a thorny problem, Román-Leshkov explains, because the long-chain molecules in plastics are held together by carbon bonds, which are “very stable and difficult to break apart.” Existing techniques for breaking these bonds tend to produce a random mix of different molecules, which would then require complex refining methods to separate out into usable specific compounds. “The problem is,” he says, “there’s no way to control where in the carbon chain you break the molecule.”
But to the surprise of the researchers, a catalyst made of a microporous material called a zeolite that contains cobalt nanoparticles can selectively break down various plastic polymer molecules and turn more than 80 percent of them into propane.
Although zeolites are riddled with tiny pores less than a nanometer wide (corresponding to the width of the polymer chains), a logical assumption had been that there would be little interaction at all between the zeolite and the polymers. Surprisingly, however, the opposite turned out to be the case: Not only do the polymer chains enter the pores, but the synergistic work between cobalt and the acid sites in the zeolite can break the chain at the same point. That cleavage site turned out to correspond to chopping off exactly one propane molecule without generating unwanted methane, leaving the rest of the longer hydrocarbons ready to undergo the process, again and again.
“Once you have this one compound, propane, you lessen the burden on downstream separations,” Román-Leshkov says. “That’s the essence of why we think this is quite important. We’re not only breaking the bonds, but we’re generating mainly a single product” that can be used for many different products and processes.
The materials needed for the process, zeolites and cobalt, “are both quite cheap” and widely available, he says, although today most cobalt comes from troubled areas in the Democratic Republic of Congo. Some new production is being developed in Canada, Cuba, and other places. The other material needed for the process is hydrogen, which today is mostly produced from fossil fuels but can easily be made other ways, including electrolysis of water using carbon-free electricity such as solar or wind power.
The researchers tested their system on a real example of mixed recycled plastic, producing promising results. But more testing will be needed on a greater variety of mixed waste streams to determine how much fouling takes place from various contaminants in the material — such as inks, glues, and labels attached to the plastic containers, or other nonplastic materials that get mixed in with the waste — and how that affects the long-term stability of the process.
Together with collaborators at NREL, the MIT team is also continuing to study the economics of the system, and analyzing how it can fit into today’s systems for handling plastic and mixed waste streams. “We don’t have all the answers yet,” Román-Leshkov says, but preliminary analysis looks promising.
The research team included Amani Ebrahim and Simone Bare at the SLAC National Accelerator Laboratory; Jie Zhu, Anna Brenner, Griffin Drake and Julie Rorrer at MIT; and Greg Beckham at the National Renewable Energy Laboratory. The work was supported by the U.S. Department of Energy (DoE), the Swiss National Science Foundation, and the DoE’s Office of Energy Efficiency and Renewable Energy, Advanced Manufacturing Office (AMO), and Bioenergy Technologies Office (BETO), as part of the the Bio-Optimized Technologies to keep Thermoplastics out of Landfills and the Environment (BOTTLE) Consortium.